Will Your Lab be Ready for the Next Public Health Emergency?
3/24/2021
When the COVID-19 pandemic hit full-force during March of 2020 and emergency use authorized (EUA) tests became available for measuring SARS-CoV-2, was your laboratory ready? Did you know what you needed to do before implementing the new tests? At CLSI, we know that time period was (and may still be) difficult and stressful for your laboratory, with many unknowns. To help you prepare for the next emergency event, we brought together a working group of experts to consolidate information on implementing such new laboratory tests. Designated as EP431 “Implementing a Laboratory Test Under Emergency Use Conditions,” expected for publication later this year, this new white paper is expected to contain a great deal of useful information for your laboratory.
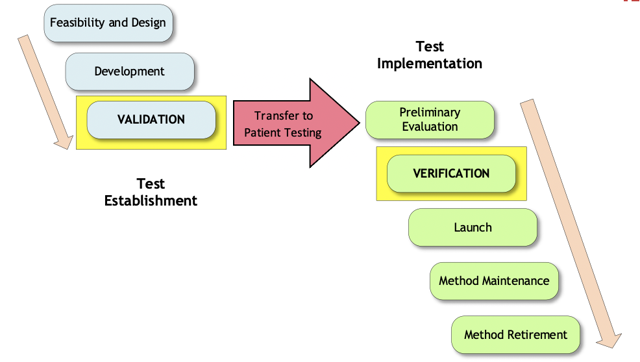
Because public health emergencies are infrequent, and because each public health emergency may have different rules and conditions that may change over time, it’s always important for the laboratory to check the websites of regulators and accreditors for the latest information. As examples in the white paper, the working group compared and contrasted the recent Ebola and COVID-19 emergency requirements. In any emergency situation however, there are basic steps to prepare for, implement, and maintain the EUA test. The new EP43 white paper will list many of these steps, following the Test Life Phase Model (Figure 1). The figure shows the two main stages of test development, Test Establishment and Test Implementation. Within each of these stages, the various phases are shown.
Figure 1. Test Life Phase Model
The test developer is responsible for the phases on the “Test Establishment” side, and the laboratory is responsible for the phases on the “Test Implementation” side. Some very real challenges may present themselves as the laboratory attempts to proceed through the test implementation phases. Many factors need to be considered before beginning testing, such as:
- Which test(s) to select.
- Likely impact on laboratory test volumes, operational hours, staffing, and cross-training.
- Reagent and consumables availability.
- Reporting requirements to local, state, or federal authorities.
- LIS and/or IT needs.
- Biosafety and biosecurity risk assessments.
Once the test is within the laboratory, additional concerns may be realized, such as:
- Availability of positive specimens for verification testing.
- Determining protocols for verification testing (especially given limited information in the product labeling).
- Training and qualification needs.
- Confirmatory testing of positives, if needed.
- Billing and reimbursement.
- Evolving requirements.
After all the hard work of planning, implementing, and using the EUA test on a daily basis, it may or may not receive regulatory clearance or approval for routine use. In either case, it will cease to be covered by the EUA. At that point in time, it will be important to ensure that the original verification studies are within the parameters of the cleared or approved product labeling. If not, new verification studies may be needed, or the test may need to be retired from use and replaced with a different approved or cleared test, starting the process all over again.
As always, you can count on CLSI to have the verification procedures you need in the Method Evaluations series of standards. You can find a list of these helpful documents here.
1 CLSI. Implementing a Laboratory Test Under Emergency Use Conditions. 1st ed. CLSI white paper EP43. Clinical and Laboratory Standards Institute; In development.
