Re-Exploring the Intermediate Interpretive Category
4/26/2021
Romney M. Humphries, PhD, D(ABMM), Vanderbilt University Medical Center, Nashville, TN
Clinical breakpoints provide an interpretation of the probability of treatment success, based on the MIC value or the area of growth inhibition by disk diffusion. Isolates with results within the susceptible category are predicted to be associated with a high chance of treatment success when the patient is administered that antimicrobial, whereas those in the resistant category are associated with low chance of treatment success (see Figure 1). Factors that improve the chances of treatment success include the dosing regimen and the concentration of the antimicrobial at the site of infection. These variables make defining a single MIC or zone diameter cut-off value for a susceptible or a resistant result that applies to all infections and dosing regimens extremely difficult. This challenge is amplified by the inherent variability of susceptibility tests as MIC values are only reproducible within +/- 1 log2 dilution.
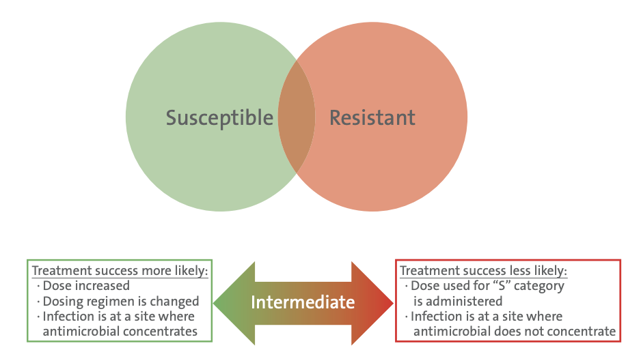
Figure 1 (Right). Implication of the Intermediate Interpretive Category
CLSI traditionally applied the intermediate category to address these challenges. Various uses for the intermediate category include:
- Provide flexibility when variable dosing regimens are possible for an antimicrobial. In this case, “I” means increasing the dose may improve the chance of treatment success.
- Acknowledge that at some anatomical sites, the antimicrobial is more concentrated. In this case, “I” means that if the infection is restricted to that site, success is likely (eg, urinary tract infections for many antimicrobials that are renally excreted).
- Provide a buffer zone between susceptible and resistant categories to prevent resistant isolates from being incorrectly categorrized as susceptible, or vice versa. Historically, CLSI did not clearly define which of the above “I” definitions applied to which breakpoints, leaving some uncertainty on how to best interpret results reported as intermediate. In practice, many clinicians interpret an intermediate result to mean resistant, when in fact in some instances it may indicate susceptible.
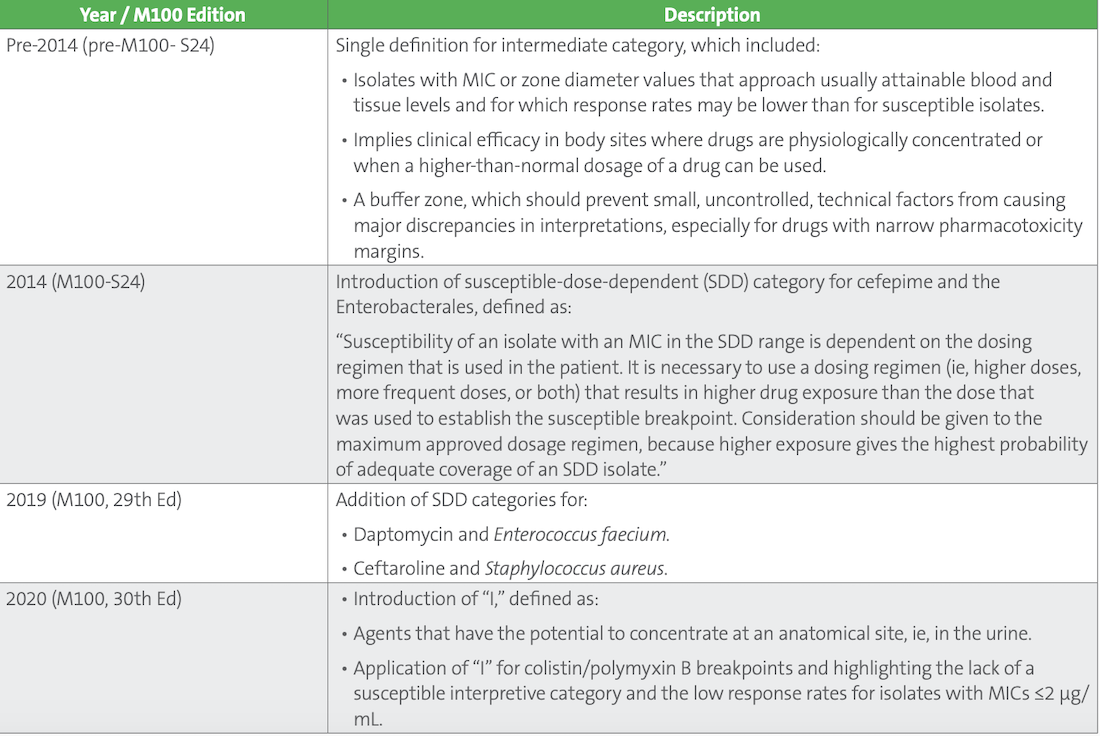
Over the past several years, CLSI has reevaluated the intermediate category and subsequently added two new categories: susceptible dose-dependent (SDD) and “I^” (Table 1). SDD was introduced to M100 in 2014 to provide clarity on when alternative dosing may be possible. In 2020, “I^” was added to highlight those antimicrobial agents that concentrate in urine and the likelihood of treatment success when the agent is prescribed for uncomplicated urinary tract infections. In addition, in 2020 the intermediate category was adapted for colistin and polymyxin B to highlight the low response rates associated with these antimicrobials. In this case, no susceptible category exits, just intermediate and resistant categories.
Table 1. Evolution of the Intermediate Category
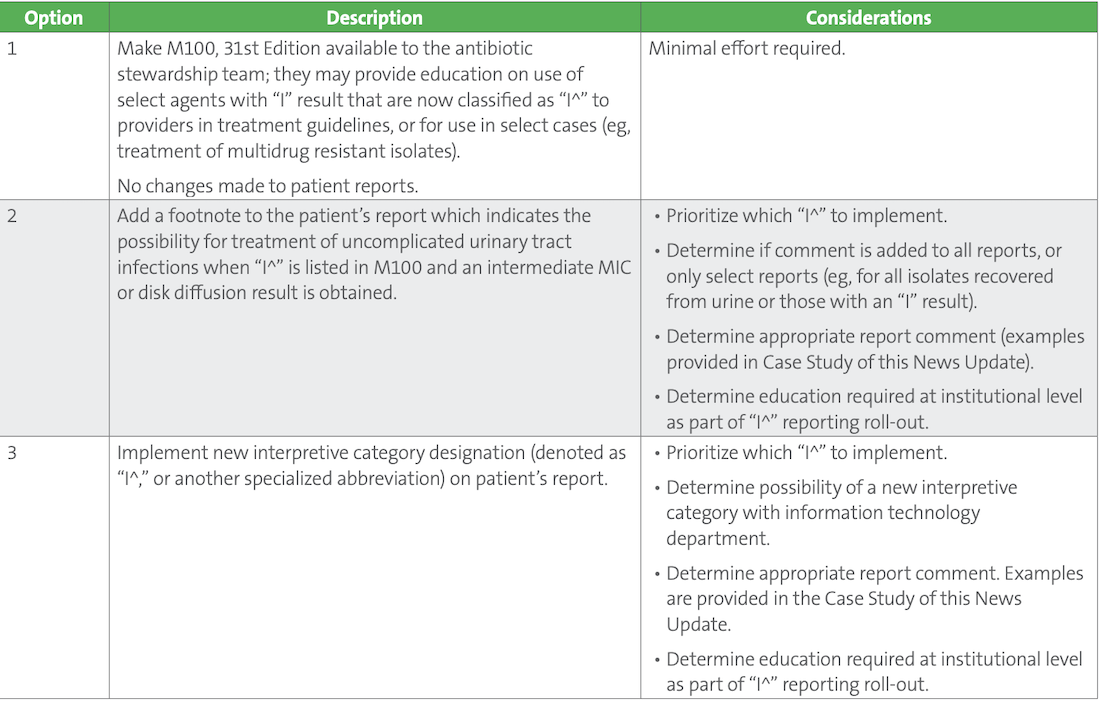
The US Food and Drug Administration (FDA) does not presently recognize either SDD or “I^,” which means commercial test systems cannot achieve FDA clearance using these expanded categories and must continue to apply the undifferentiated intermediate category, which includes SDD, “I,” and “I^.” As such, implementation of SDD and “I^” categories by clinical laboratories is complex, requiring information technology (IT) solutions and careful consideration as to which (if any) of these categories would provide a significant impact to optimal patient treatment. The laboratory should discuss how to prioritize implementation of SDD and “I^” categories with the antimicrobial stewardship team, pharmacy, infectious diseases clinicians, information technology experts, and other vested stakeholders. Implementation of “I^” specifically can be approached in a variety of ways, as described in Table 2. Of note, because “I^” merely reflects a change to the interpretive category designation and not to the breakpoint, validation of a laboratory’s susceptibility test system is not needed outside of validating IT changes and results reporting. The options listed in Table 2. are not mutually exclusive, and laboratories may opt for multiple approaches, in a stepwise manner.
Table 2. Options for Implementation of “I^”
This article came from AST News Update, Volume 6, Issue 1 – April 2021 which is produced by the CLSI Outreach Working Group (ORWG). The ORWG is part of the CLSI Subcommittee on Antimicrobial Susceptibility Testing (AST) and was established in 2015. The formation of the working group originated in a desire to efficiently convey information regarding contemporary AST practices, recommendations, and resources to the clinical microbiology community. They welcome suggestions from you about any aspect of CLSI documents, educational materials, or their Newsletters.
