Learning About Veterinary AST From Lady Glittersparkles
8/31/2020

Robert Bowden, Beth Israel Deaconess Medical Center, Boston, MA Claire Burbick, Washington Animal Disease Diagnostic Lab, Washington State University, Pullman, MA
Just as the CLSI subcommittee on AST develops standards that support the judicious use of antimicrobial agents in human medicine, the subcommittee on Veterinary Antimicrobial Susceptibility Testing (VAST) develops standards for performing AST on bacteria isolated from animals and releases VET documents (listed in Table 1) that closely parallel the CLSI “M” series of AST documents.
Case Study Table 1. CLSI Veterinary AST Documents

CLSI VAST Documents VET01 and VET03 describe methodology for performing AST (broth dilution, disk diffusion, and agar dilution), while VET04, VET06, and VET01S provide tables with quality control parameters and veterinary-specific clinical breakpoints for dogs, cats, cattle, horses, pigs, poultry, and fish. Other important groups, such as birds, sheep, goats, camelids, reptiles, amphibians, and wild animals, currently lack breakpoints due to limited clinical and pharmacologic data, though antimicrobials are used to treat these animal groups and are, in some cases, necessary for endangered species facing population-limiting bacterial or fungal infections. For small companion animals, however, VAST has determined veterinary-specific breakpoints for a broad variety of antimicrobials. Many of these agents, and many of the pathogens commonly infecting dogs and cats, will be quite familiar to colleagues working in human medicine. Furthermore, a new educational document, VET09, exists to aid veterinarians and clinical laboratories in understanding important concepts in AST of veterinary isolates.
To illustrate an approach to AST of veterinary isolates, and to examine the similarities and differences between AST workup in veterinary and human labs, we will look at the case of a canine patient presenting at a small private veterinary clinic.
The Case
An 11-year-old female spayed German Shepherd, Lady Glittersparkles, is brought to a primary care veterinary clinic with the owner reporting that the patient has exhibited a two-day history of increased urinary frequency and straining, along with several accidents in the house. Lady has a history of controlled diabetes mellitus and chronic kidney disease.
A cystocentesis (ie, removal of fluid from the bladder) is performed and sent to the diagnostic laboratory at the state’s veterinary academic teaching hospital for urinalysis and culture.
Veterinary microbiology testing is most often done at commercial reference labs, veterinary teaching hospitals associated with veterinary colleges, or state-supported veterinary diagnostic laboratories. Very few clinics perform microbiology testing in-house.
Lady receives an empiric 10-day prescription for the veterinary formulation of oral amoxicillin-clavulanate at the labeled dosage for dogs of 13.75 mg/ kg every 12 hours.1 Longer treatment durations remain common practice in veterinary medicine, as there is little veterinary-specific evidence for short course therapy, and practitioners’ awareness of this evolution in human medicine is limited.2 Beta-lactams and fluoroquinolones are the most common agents prescribed for urinary tract infections (UTIs) in dogs, and drugs commonly utilized in human medicine, like nitrofurantoin and trimethoprim-sulfamethoxazole, are infrequently used. Currently, no veterinary breakpoints exist for nitrofurantoin or trimethoprim-sulfamethoxazole.3 Many veterinarians are unfamiliar with the former and are reluctant to use the latter particularly in certain large breed dogs such as shepherds due to concerns about their genetic predisposition for idiosyncratic reactions, though the degree of risk remains controversial.
Small animal veterinary medicine relies almost exclusively on oral agents or long-acting single-dose subcutaneous injectables due either to the difficulty or expense of outpatient parenteral antimicrobial therapy (OPAT) administration, or the difficulty in administering oral medication to unruly pets. Furthermore, as expenses are paid out of pocket by owners, the type and extent of diagnostic testing that may be performed and cost of prescriptions must also be taken into consideration. For all these reasons, veterinary fluoroquinolones (enrofloxacin, orbifloxacin, marbofloxacin, and pradofloxacin) or third-generation cephalosporins (cefpodoxime) are frequently utilized, especially for cats because of the greater ease afforded by once-daily dosing. A long-acting, injectable 3rd-generation cephalosporin, cefovecin, is also frequently used to treat UTIs in cats when oral administration is not possible. (Note: these descriptions are not intended as a position statement on antimicrobial selection or stewardship but are included to provide insight into several of the factors surrounding testing and use of antimicrobial agents which are unique to veterinary medicine.)
A standard quantitative culture is performed at the microbiology laboratory, where 1 μL of urine is inoculated on each half of a blood/MacConkey agar biplate, and 10 μL is plated to a separate blood agar plate. After overnight incubation at 35°C in 5% CO2 , a single colony morphology is observed and quantitated as >100,000 CFU/mL. The isolate is a gram-negative rod that is betahemolytic, lactose-fermenting, indole-positive, and oxidase-negative, allowing for an at-the-bench identification of E. coli.
For less straightforward identifications, many veterinary laboratories still rely on biochemical methods, though acquisition and utilization of MALDI-TOF MS is increasing.
As is true for humans, E. coli is the most common cause of UTI in both dogs and cats. Other species commonly implicated in UTIs will also be familiar, including staphylococci (most often S. pseudintermedius in dogs and S. felis in cats), enterococci, and somewhat less frequently, Proteus mirabilis and Klebsiella pneumoniae.
One very notable difference between human and veterinary AST is that the FDA does not regulate AST devices for veterinary use, nor does it set veterinary breakpoints.
Veterinary AST is most often performed using commercial broth microdilution MIC methods with panels designed specifically for veterinary medicine.
Disk diffusion testing is less common as there are no disk diffusion breakpoints for many of the agents that might be tested in a veterinary laboratory. This is usually due to an absence of disk correlate data at the time breakpoints were established or the result of poor correlation between MIC and disk methods. In the current case, the laboratory tests the isolate using a commercial Gram negative broth microdilution panel for companion animals, yielding the results shown in Table 2.
Table 2. AST results for E. coli urine isolate
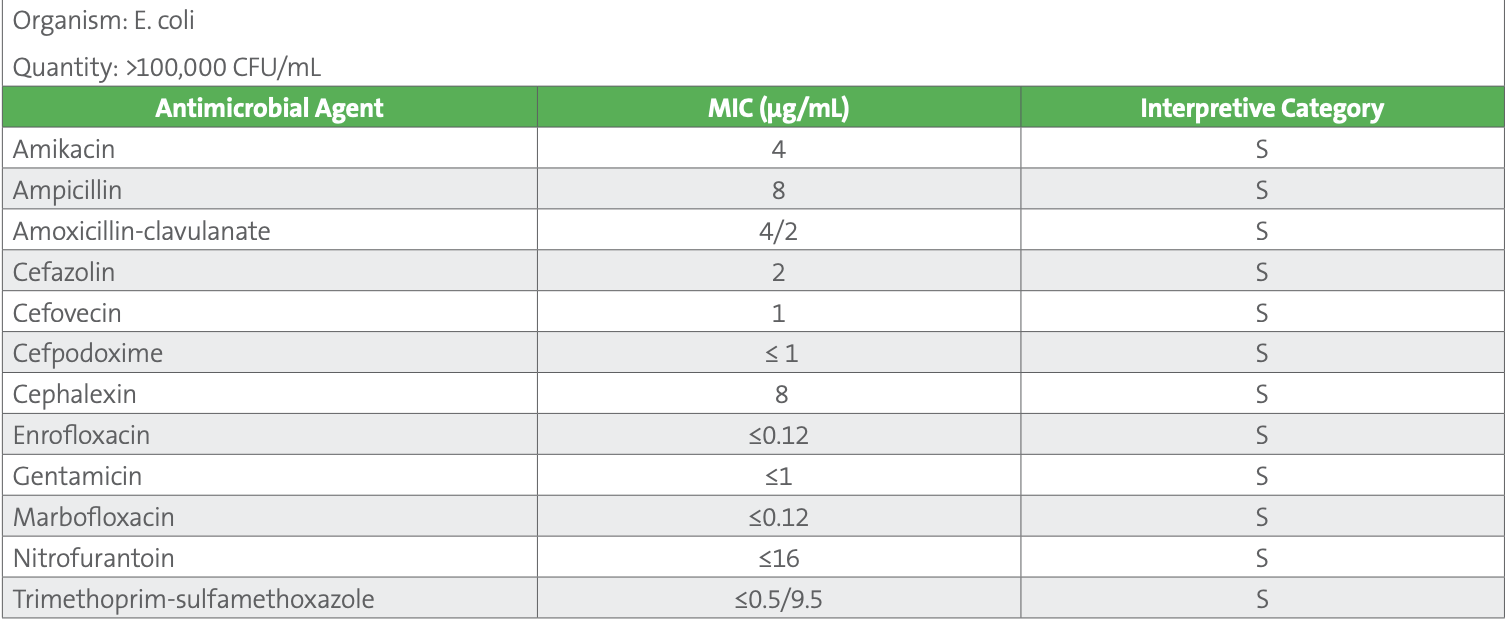
Note: Additional agents tested on the panel but not reported include (µg/mL): ceftazidime ≤1 S, chloramphenicol 8 S, imipenem ≤1 S, piperacillin-tazobactam 2/4 S
For most of these agents, canine-specific breakpoints are published in Table 2A of VET01S. Agents of interest for which canine-specific breakpoints have not been published are generally interpreted using human breakpoints from M100. These are reproduced in VET08 and designated as human breakpoints, with the recognition that they may be less predictive of clinical outcomes as compared to veterinary-specific breakpoints. They include chloramphenicol, imipenem, nitrofurantoin, and trimethoprim-sulfamethoxazole. Many veterinary laboratories routinely suppress results for chloramphenicol and carbapenems, and their use is prohibited in some countries, although these agents may be reported and considered for use in some countries when few other options exist. Additionally, canine-specific breakpoints for ceftazidime and piperacillin-tazobactam are listed as secondary agents, to be selectively reported. Table 3 shows an example of how veterinary-specific and human breakpoints are presented in VET08- Ed4.
Table 3. Excerpt from CLSI VET08-Ed4

For cefovecin, there are dog-specific and cat-specific breakpoints. At this time, human breakpoints (denoted with grey shading) must be applied for interpreting trimethoprim-sulfamethoxazole on veterinary isolates, as veterinary breakpoints have not been established.
Summary
This case is simple but intended to demonstrate the value of CLSI VET documents in providing veterinary laboratories with standards that assure high quality AST results are produced. It also shows the overarching similarities between the work of the human and veterinary subcommittees on AST.
The E. coli encountered here is pan-susceptible, and the empiric therapy is likely to bring resolution. Increasingly, though, many cases are less straightforward. Advances in veterinary medicine over the past 20 years enable treatments that often parallel interventions and therapies seen in human medicine. Orthopedic surgery, radiation oncology, and dermatology are just a few of the specialties that have greatly extended and improved quality of life for veterinary patients. As is true in human medicine, however, opportunistic infections and resistance phenotypes that were previously rare are now encountered with more regularity in small animal medicine, including oxacillin-resistant staphylococci causing skin, urinary tract, and even prosthetic joint infections in dogs,4 multidrug-resistant Pseudomonas aeruginosa from chronic ear infections in dogs, and ESBL-producing Enterobacterales in multiple species. These challenges highlight the necessary work of VAST as it continues to develop veterinary-specific criteria that assist appropriate selection of antimicrobial agents in veterinary medicine.
References
1 Clavamox® [package insert]. Kalamazoo, MI. Zoetis Inc. 2013. Accessed July 5, 2020.
2 Weese JS, Blondeau J, Boothe D, et al. Antimicrobial use guidelines for treatment of urinary tract infections in dogs and cats: Antimicrobial guidelines working group of the International Society for Companion Animal Infectious Diseases. Vet Med Int 2011;4:1–9.
3 CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. 4th ed. CLSI supplement VET08. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
4 Międzobrodzki J, Kasprowicz A, Białecka A, Jaworska O, Polakowska K, Władyka B, Dubin A. The first case of a Staphylococcus pseudintermedius infection after joint prosthesis implantation in a dog. Pol J Microbiol. 2010;59:133–35.
