AST News Update January 2024: Antifungal Body Site Reporting for Candida spp.
1/22/2024

Amir Seyedmousavi, National Institutes of Health, Bethesda, MD
Audrey N. Schuetz, Mayo Clinic, Rochester, MN
A 59-year-old woman with metastatic breast cancer was admitted to a hospital for fever and suspected sepsis 12 days after chemotherapy-induced neutropenia (<1,000 neutrophils/μL in peripheral blood; normal range 2500-7000 neutrophils/μL). The patient was started empirically on cefepime, vancomycin, and anidulafungin. Two sets of blood cultures grew Candida tropicalis on day 3 post admission. Since this isolate was recovered from the blood, both species-level identification and susceptibility testing were performed. At the time of blood culture positivity, the patient did not report any visual symptoms, and dilated fundoscopy of the eyes on day 4 did not reveal any signs of ocular involvement.
Table 1. summarizes the antifungal susceptibility test results on the C. tropicalis blood culture isolate using a commercial broth microdilution method. Minimal inhibitory concentrations (MICs) were interpreted according to breakpoints and epidemiological cutoff values (ECVs) available in CLSI M27M44S-ED3:2022 and CLSI M57S-ED4:2022, respectively,1, 2 which showed susceptibility to all echinocandin antifungal agents (anidulafungin, caspofungin, and micafungin) with an MIC of 0.06 μg/mL to each.
Table 1. Antifungal Susceptibility Test Results of Candida tropicalis Isolated From Blood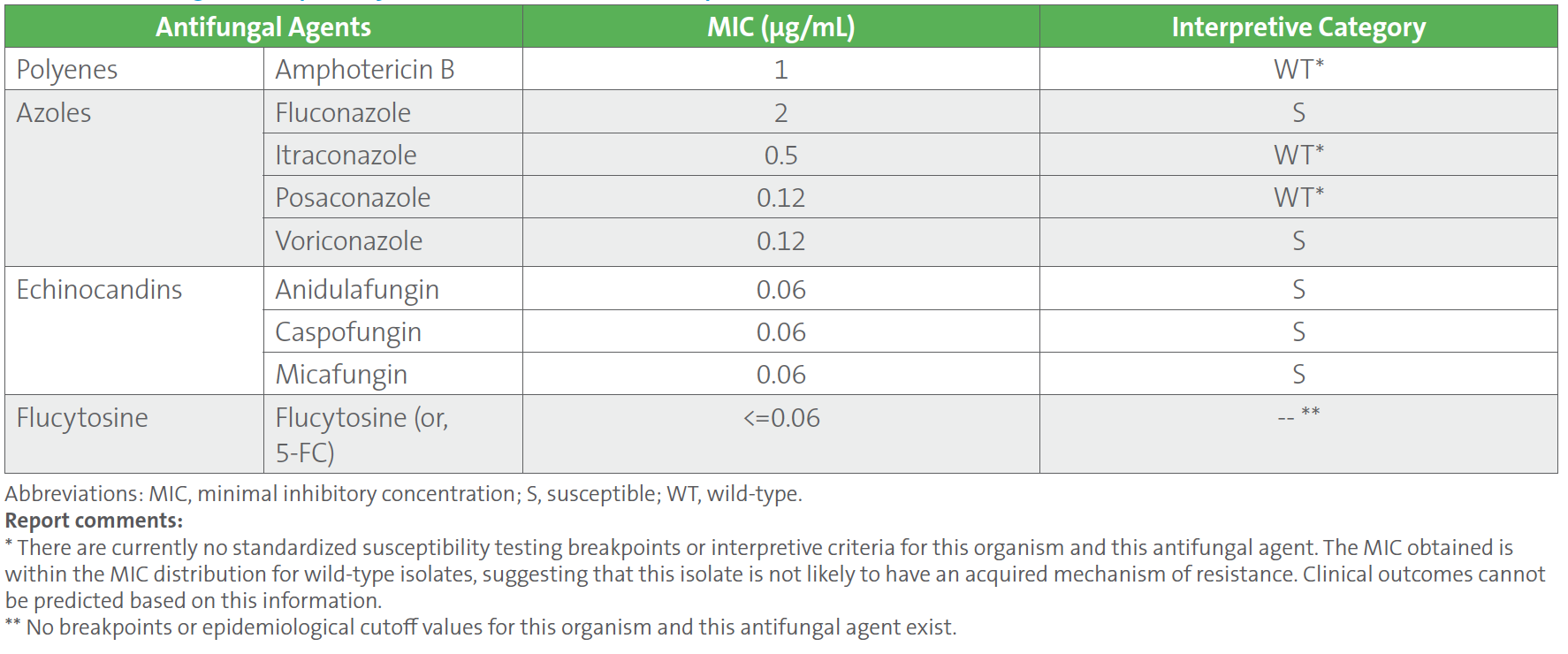
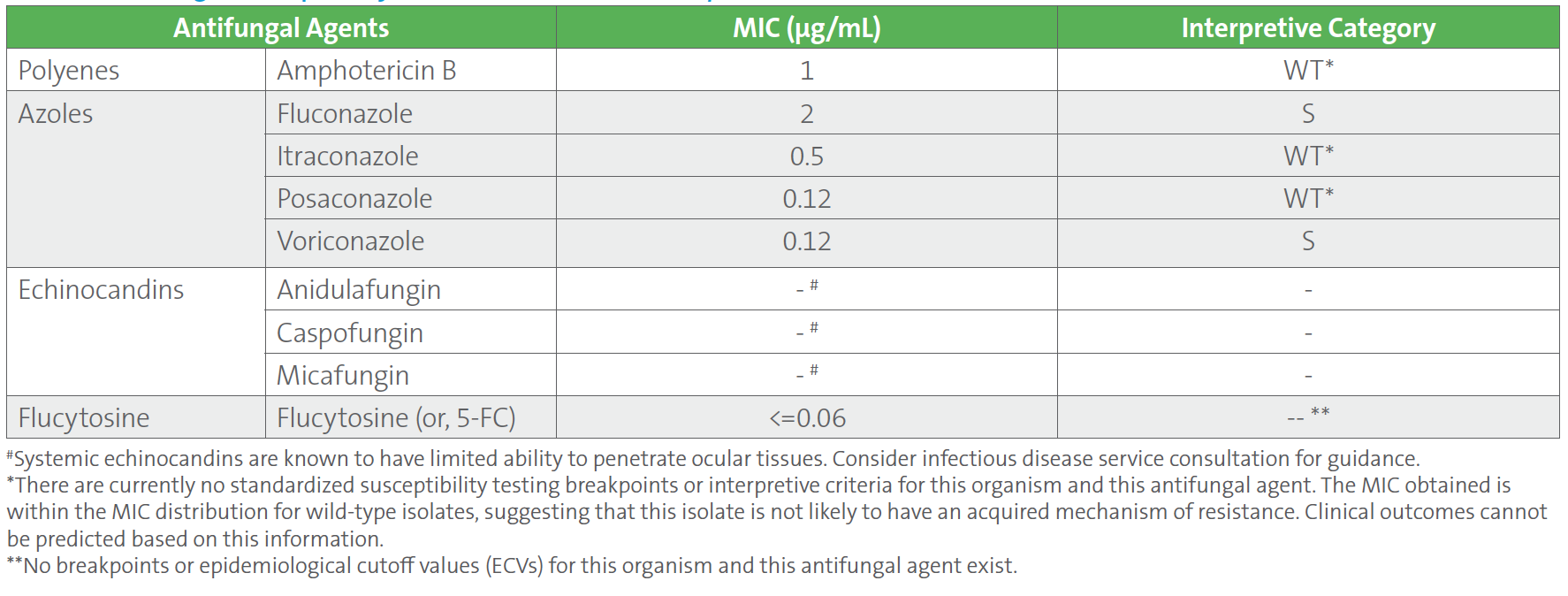
Repeat blood cultures drawn 4 days after the initial positive blood cultures were negative. The patient was improving clinically on cefepime, vancomycin, and anidulafungin. However, ten days into treatment, the patient developed progressive blurring of vision in the right eye. Ophthalmic fundoscopy showed a low visual acuity of 20/200, marked vitreous exudates, and prominent areas of chorioretinitis, all of which confirm endophthalmitis (ie, inflammation of the inner parts of the eye). Cultures of blood and vitreous fluid were collected. Blood cultures were negative, but vitreous fluid cultures grew C. tropicalis. Antifungal susceptibility testing was performed on the C. tropicalis isolated from vitreous fluid, and the results showed identical MIC values to the initial blood culture isolate. However, the MIC values for all three echinocandins (anidulafungin, caspofungin, and micafungin) were suppressed from the final report (Table 2), according to CLSI M27M44S-ED3:2022 guidelines, since the isolate was ocular in origin.1 Based on the susceptibility results and site of infection, anidulafungin was stopped and switched to intravenous liposomal amphotericin B and voriconazole for 6 days, followed by oral fluconazole. She also received amphotericin B by intravitreal administration (ie, injected directly into the eye) and underwent vitrectomy.
Table 2. Antifungal Susceptibility Test Results of Candida tropicalis Isolated From Vitreous Fluid
Should echinocandins be reported on Candida isolates from ocular specimens? If so, how?
Case study answer and discussion:
Candida species are common causes of fungal endophthalmitis. This infection arises from either endogenous or exogenous sources. The endogenous form most often occurs following candidemia via hematogenous spread to the eye, with infection typically progressing through the retina at the back of the eyeball into the vitreous fluid. Exogenous endophthalmitis is usually a consequence of trauma, eye surgery, or progression of corneal infection (ie, fungal keratitis). Successful therapy of Candida endophthalmitis requires penetration of antifungal agent into the relevant compartments of the eye (ie, the choroid, retina, vitreous fluid, and aqueous humor).
In the current case, the patient developed endogenous C. tropicalis endophthalmitis while receiving anidulafungin therapy. Echinocandin antifungal agents are highly active and fungicidal against Candida spp., including isolates that are resistant to triazoles and species that form biofilms. They are first line agents for candidemia. The Infectious Diseases Society of America (IDSA) also recommends performance of a dilated retinal exam during the first week of treatment in cases of candidemia due to risk of seeding the eye, since evidence of hematogenous spread of Candida to the eye may impact choice of antifungal therapy.3
Ocular candidiasis can be treated with systemic antifungal therapy with intravitreal injection of an antifungal agent, sometimes combined with vitrectomy.3, 4 Understanding how antifungal agents penetrate in ocular tissue (cornea, aqueous, vitreous) is a critical factor to achieving optimal outcome for endogenous Candida endophthalmitis.5
Amphotericin B has been used successfully for the treatment of invasive orbital and intraocular infections. Penetration of amphotericin B into the eyes is also enhanced by inflammation. Therefore, intravenous administration combined with direct injection of amphotericin B into the eye is the recommended route of administration in patients with severe endophthalmitis.
Among azoles, voriconazole is the ideal choice in the treatment of ocular fungal infections, as it has a high intraocular penetration profile and a broad spectrum of activity against all Candida species.5 Fluconazole also achieves high levels of penetration (25-100% of the plasma concentration) into ocular tissues within hours after a single dose.6 Voriconazole and fluconazole administered systemically are detectable in aqueous and vitreous fluids of uninflamed and inflamed eyes. Most published clinical experience of Candida endophthalmitis describes the use of voriconazole and fluconazole.7 Ocular penetration of itraconazole and posaconazole appears to be low, and little or no data exist for isavuconazole.
All three echinocandin antifungal agents currently approved by the United States Food and Drug Administration (FDA) to treat candidemia show limited penetration into the eye due to their large molecular weight. After systemic administration, the echinocandins distribute well into major tissues, including lung, liver, and spleen. However, they achieve undetectable or very low vitreous concentrations relative to plasma.4 The subtherapeutic penetration into ocular tissues has been associated with treatment failure in Candida endophthalmitis.
Candida species isolated from sterile sites (eg, blood, cerebral spinal fluid, joint fluid, pleural fluid, pericardial fluid, and ocular tissue) are recommended to routinely undergo species-level identification and susceptibility testing. For a proper appreciation of antifungal efficacy in different tissues, the CLSI M27M44S-ED3:2022 and CLSI M57S-ED4:2022 guidelines,1,2 provide recommendations on how to report susceptibility results into the patient record for situations during which Candida is isolated from different anatomical sites (Table 3). Body site-specific guidelines for Candida reporting are provided for azoles and the echinocandins for ocular sources including cornea, aqueous humor, and vitreous fluid. Other body site sources which are covered in the CLSI documents include urine for amphotericin B, azoles, and echinocandins; cerebrospinal fluid, central nervous system tissue, and abscess material for azoles and echinocandins. No reporting restrictions are proposed for flucytosine.
Table 3. CLSI Recommendations on Reporting Antifungal Susceptibility Test Results for Candida spp. Isolated From Ocular Specimens1,2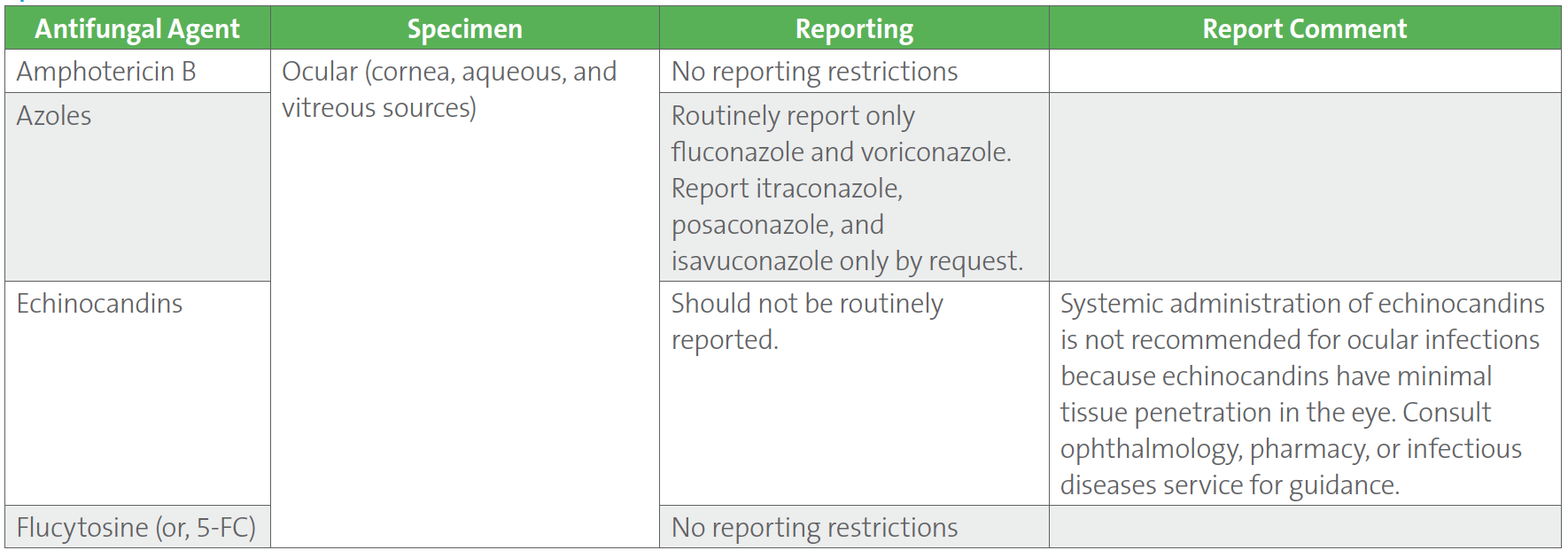
Case follow-up:
The choice of antifungal agent(s) for treatment depends on the susceptibility profile of the Candida spp. isolate as well as penetration to anatomical site of infection. Based on the MIC breakpoints, Candida spp. may demonstrate susceptibility or resistance to multiple classes of antifungal drugs. In addition, the possibility of a difference in antifungal susceptibility pattern when the same isolate is isolated from different body sites (ie, blood and vitreous fluid) also supports performance of antifungal susceptibility testing of Candida when isolated from multiple sterile sites.
In the current case, the patient was initially begun on an echinocandin for candidemia and underwent an eye exam during the first week of candidemia treatment, as suggested by IDSA, at which time there was no evidence of ocular involvement. However, the patient experienced visual symptoms on day 10 and was confirmed to have developed Candida endophthalmitis while on treatment with an echinocandin. Given the positive eye cultures, treatment was changed to systemic liposomal amphotericin B and voriconazole as well as intravitreal amphotericin B. Vitrectomy combined with systemic and intravitreal antifungal treatment yielded a favorable outcome in the management of endogenous endophthalmitis due to C. tropicalis in the current case.
In summary, evidence from IDSA suggests that echinocandins are appropriate initial therapy for candidemia, and eye examinations should be performed during the initial week of candidemia therapy to assess whether endophthalmitis is present. If endophthalmitis is present, echinocandins are not the appropriate choice, given that they do not penetrate ocular tissues due to their large molecular weight. For the management of sight-threatening lesions in the eye, achieving adequate concentrations of the appropriate antifungal agent in the area of the eye that is infected is crucial to success. The intravitreal injection of amphotericin B or voriconazole is helpful to achieve high local antifungal activity as quickly as possible because these agents achieve adequate concentrations in the posterior part of the eye and within the vitreous fluid. The antifungal susceptibility pattern of the infecting Candida species is also important to assess, since multidrug resistance has been noted for many Candida species and raises additional treatment challenges.4
References
1. CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts. 3rd ed. CLSI supplement M27M44S. Clinical and Laboratory Standards Institute; 2022.
2. CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing. 4th ed. CLSI supplement M57S. Clinical and Laboratory Standards Institute; 2022.
3. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infec Dis. 2016;62(4)e1–e50.
4. Oude Lashof AML, Rothova A, Sobel JD, et al. Ocular Manifestations of Candidemia. Clin Infec Dis. 2011;53(3)262–268.
5. Felton T, Troke PF, Hope WW. Tissue Penetration of Antifungal Agents. Clin Microbiol Rev. 2014;(27):68-88.
6. O’Day DM, Foulds G, Williams TE, Robinson RD, Allen RH, Head WS. Ocular uptake of fluconazole following oral administration. Arch Ophthalmol. 1990;(108)1006-8.
7. Danielescu C, Stanca HT, Iorga RE, Darabus DM, Potop V. The Diagnosis and Treatment of Fungal Endophthalmitis: An Update. Diagnostics (Basel). 2022;12(3)679.
