When Should Antifungal Susceptibility Testing Be Performed For Candida Species Isolated From Clinical Specimens?
2/13/2019
Written By: Cecilia Carvalhaes, JMI Laboratories, North Liberty, Iowa
Background
There has been a greater demand for antifungal susceptibility testing (AFST) in recent years as a result of the increased use of antifungals, the recognition of innate resistance in some fungal species, and the emergence of resistance during therapy.1 AFST results can be useful to:
- Predict the likely outcome and guide antifungal therapy in specific clinical circumstances, including candidemia and mucosal candidiasis.2,3
- Detect resistance that develops during therapy
- Monitor local susceptibility patterns that will guide empiric therapy
Although Candida spp. are the most prevalent organisms associated with invasive fungal infections, each species has its unique virulence potential, antifungal susceptibility patterns, and epidemiology.4 Of note, Candida auris, a pathogen first described in 2009 that has reduced susceptibility to the major antifungal classes, has emerged as a cause of rapidly spreading nosocomial outbreaks associated with high mortality rates.5,6
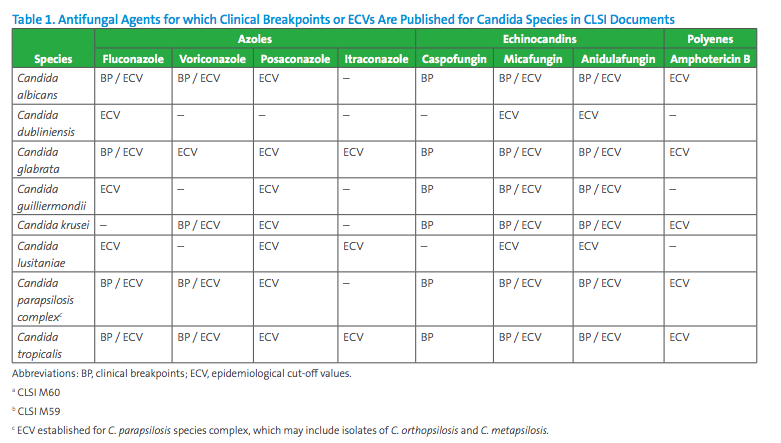
In 2017, CLSI combined broth and disk susceptibility testing recommendations for Candida and species-specific MIC interpretive breakpoints into CLSI document M60.7 This document includes breakpoints for the most frequent Candida species for selected agents (echinocandins, fluconazole, and voriconazole, see Table 1). Note that not all Candida species are represented in CLSI document M60 and breakpoints have not been set for all antifungal agents that may warrant testing. In the absence of breakpoints, the epidemiological cutoff value (ECV) published in CLSI document M59 is useful to alert for the possibility of intrinsic or acquired resistance based on the MIC value (see Table 1).9 ECV is the MIC value that presumptively separates populations into those with and without intrinsic or acquired resistance traits. ECVs are not meant to predict the clinical outcome to therapy, and a susceptibility category (eg, Susceptible/ Intermediate/ Susceptible-Dose Dependent/ Resistant) should not be reported with an ECV since the value does not take into consideration clinical or pharmacodynamic and pharmacokinetic data. However, ECVs should help to detect those strains that are likely to harbor antifungal resistance mechanisms.8
The Infectious Diseases Society of America (IDSA) and the European Society of Clinical Microbiology and Infectious Disease recommend routine antifungal susceptibility testing for all clinically relevant Candida isolates. Clinically relevant isolates are those recovered from blood and other sterile body sites, including tissue biopsies, bone, and fluids, such as cerebrospinal, peritoneal, pericardial, pleural, and synovial fluid.2,3 Candida spp. isolates recovered from body sites where colonization is common, such as respiratory tract, gastrointestinal tract, vagina or skin should not be considered clinically relevant and susceptibility testing is generally not performed for isolates from these sites.
This article addresses the susceptibility testing for Candida species for which CLSI has established breakpoints or ECV recommendations. CLSI also offers ECVs for Cryptococcus, but such testing is not covered in this article. CLSI is also currently assessing the possibility of setting breakpoints for some antifungals for Candida auris, but those have not yet been set. Antifungal susceptibility testing of C. auris will thus be discussed in a future article.
Practical Microbiology Situations
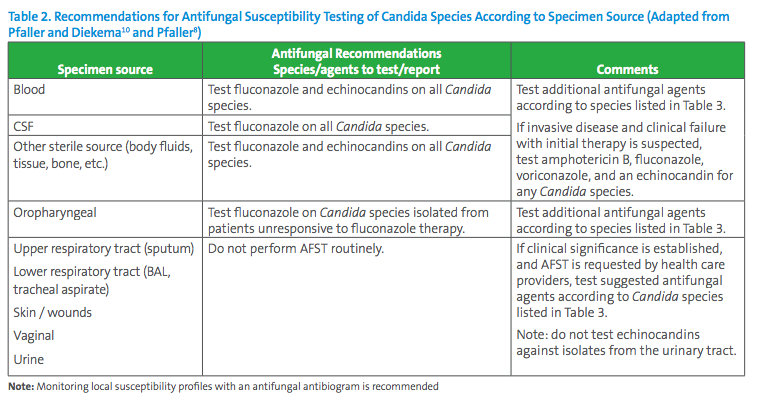
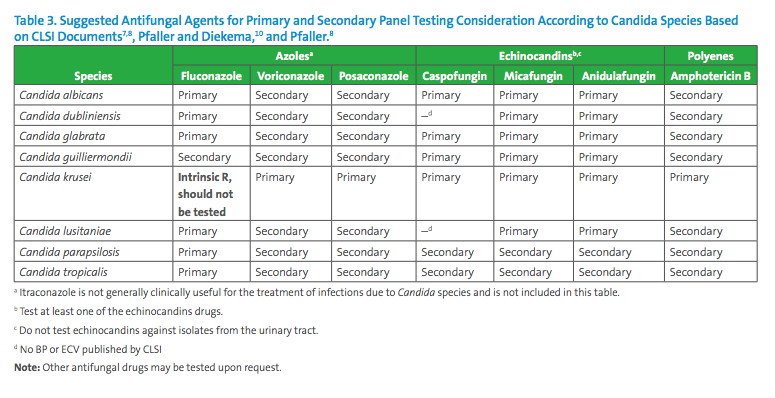
Based on published CLSI breakpoints and ECVs and literature review, Table 2 contains expert opinion guidelines for when AFST should be performed and Table 3 suggests the antifungal agents that may be considered for primary and secondary panel testing according to Candida species, also according to expert opinions published in a variety of journals.7,8,9,10 In many situations, decisions for antifungal testing must be made on a case-by-case basis with input from the microbiologist, infectious diseases practitioners and other healthcare providers.
Several examples related to antifungal testing in situations that might be encountered by clinical microbiologists are reviewed below.
1. Candida glabrata from blood culture
Discussion: Routine AFST should be performed for all Candida species from blood, at least for fluconazole. In this case, C. glabrata is a pathogen of special interest due to common acquired resistance to azoles.1 An MIC and interpretation should be reported for fluconazole and testing with echinocandins is also recommended.2,3 Since no clinical breakpoints are currently available for C. glabrata and voriconazole or posaconazole, the laboratory may consider reporting the ECVs if these agents are requested.9 Amphotericin B susceptibility may also be tested, and an ECV reported for this agent.9
2. Candida krusei from urine specimen
Discussion: If a Candida spp. is recovered from urine and is confirmed to be linked to a urinary tract infection, further workup beyond species identification is not needed. If treatment is indicated, fluconazole is usually prescribed. Echinocandins are not used to treat urinary tract infections, due to minimal excretion of active drug in the urine.2 If fluconazole susceptibility cannot be inferred by species identification, such as with C. glabrata for which acquired resistance to fluconazole is not uncommon, AFST may be warranted. And if the isolate is C. krusei which is intrinsically resistant to fluconazole, testing of posaconazole or amphotericin B may be performed (see Table 3). ECVs are available for posaconazole and amphotericin B for C. krusei (Table 1).
3. Candida albicans from oropharyngeal specimen
Discussion: Mucosal candidiasis is commonly observed in immunocompromised patients who have received antifungal prophylaxis. In these immunocompromised patients, AFST may be indicated.2 In addition, AFST would be recommended in case of treatment failure to rule out fluconazole-resistant isolates and to determine susceptibility of other agents.2,11
4. Candida albicans from vaginal specimen
Discussion: Candida albicans colonizes healthy human skin, mucosal surfaces, and the reproductive tract. Since approximately 10%–20% of women harbor Candida spp. and other yeasts in the vagina, identifying Candida by culture in the absence of symptoms or signs of infection is not an indication for treatment. However, culture for yeast remains the gold standard for diagnosis of vulvovaginal candidiasis. When treatment for clinically confirmed vulvovaginal candidiasis is required, a topical antifungal formulation containing an azole or oral fluconazole is typically prescribed empirically. In general, azole susceptibility testing is not warranted for individual treatment guidance, but fluconazole susceptibility testing may be requested for treating complicated vulvovaginal candidiasis.2,12
5. C. tropicalis from tracheal aspirate specimen
Discussion: The isolation of Candida species from the respiratory tract is commonly encountered among patients who are in the ICU and are intubated or have a chronic tracheostomy. This almost always reflects colonization of the airways and not infection. Candida tropicalis possesses a remarkable capacity to form biofilms in medical devices. AFST for C. tropicalis from tracheal aspirate is not needed.2,13
In summary, it is helpful for laboratories to have a clear approach outlining when performance of AFST is necessary and with which antifungals. Selective antifungal susceptibility testing coupled with more frequent identification of Candida to the species level has proven useful, especially in difficult-to-manage cases of invasive candidiasis.8 In addition, periodic epidemiological studies should also be done to determine the susceptibility profiles and resistance rates for individual centers.3 Finally, the use of antifungal susceptibility testing to detect emerging resistance and predict the therapeutic potential of newly discovered investigational agents is of primary importance for continuing medical progress.
References:
1 Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol. 2012;50(4):1199-1203.
2 Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-50.
3 Cuenca-Estrella M, Verweij PE, Arendrup MC, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect. 2012;18 Suppl 7:9-18.
4 Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: Report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J Clin Microbiol. 2011;49(1):396-399.
5 Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026.
6 Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64(2):134-140.
7 CLSI. Performance standards for antifungal susceptibility testing of yeasts. 1st ed. CLSI supplement M60. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
8 Pfaller MA. Invasive fungal infections and approaches to their diagnosis. In: Sails A, Tang Y, eds. Methods in Microbiology. Vol 72. Oxford: Academic Press; 2015:219-287.
9 CLSI. Epidemiological cutoff values for antifungal susceptibility testing. 2nd ed. CLSI supplement M59. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
10 Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50(9):2846-2856.
11 Patel PK, Erlandsen JE, Kirkpatrick WR, et al. The changing epidemiology of oropharyngeal candidiasis in patients with HIV/ AIDS in the era of antiretroviral therapy. AIDS Res Treat. 2012;2012:262471.
12 Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2015;64(RR-03):1-137.
13 Negri M, Silva S, Henriques M, Oliveira R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur J Clin Microbiol Infect Dis. 2012;31(7):1399-1412.
This article came from AST News Update, Volume 4, Issue 1 – January 2019 which is produced by the CLSI Outreach Working Group (ORWG). The ORWG is part of the CLSI Subcommittee on Antimicrobial Susceptibility Testing (AST) and was established in 2015. The formation of the working group originated in a desire to efficiently convey information regarding contemporary AST practices, recommendations, and resources to the clinical microbiology community. They welcome suggestions from you about any aspect of CLSI documents, educational materials, or their Newsletters.
